Science Fair Projects With Osmosis
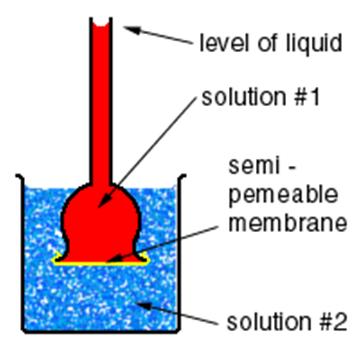
Osmosis is one of the widely discussed topics when it comes to transport of the solutions within the living things. It is responsible for a lot of important processes in human body, animals, and plants. This transport occurs through a semi permeable membrane. Demonstrating the process of osmosis is an easy task and a superb idea for your science fair project. You just have to create two mediums with various concentrations and semi permeable barrier in between. It is better to conduct this experiment in the laboratory of your school or colleges as it requires equipment easily available there.
Time Required for Setting Up the Apparatus: 10 minutes
Time Required for Making the Observations: 3 hours
Things Required:
– Beaker or transparent glass / plastic bowl
– Thistle funnel
– Concentrated sugar solution
– Distilled water
– Semi permeable membrane like parchment paper or cellophane
– Twine to secure the membrane to the mouth of the Thistle funnel
– Stand with a clamp to hold the Thistle funnel in position
– Strong twine, rubber or thread
Instructions
-
1
Take a semi-permeable membrane and place it over the mouth (opening) of the thistle-funnel. Fix it with strong twine or rubber band.
-
2
Now, carefully turn thistle-funnel upside down and fill its cup with some concentrated sugar solution.
-
3
Grab a beaker or a plastic bowl and pour some distilled water into it until filled. Now, plunge the thistle funnel with the concentrated sugar solution into it. Make sure to place the membrane-covered end the thistle funnel towards the base of your beaker.
-
4
Tightly hold the tube of the thistle funnel in an upright pose, making sure to leave some space between its covered mouth and the base of the beaker.
-
5
Pick a marker and note the level of concentrated sugar solution in the thistle funnel by marking it with your marker. Leave these equipments for some time, at least 2 to 3 hours.
-
6
Observation: Take a pen and note book and get back to the experiment after 2 to 3 hours. Note down the increase in the level of the concentrated sugar solution in the tube. Moreover, you will find the semi permeable membrane lumped out towards the bottom of the beaker.
-
7
Conclusion: The increase in the level of the concentrated sugar solution in the tube is ought to the movement of invisible water molecules into the thistle funnel. This is just because of the fact that the distilled water has a less consolidation of effort as compare to the concentrated sugar solution. As a result, the water molecules develop the tendency to travel from areas of its higher concentration into those of its lower concentration.







