Difference Between Van der Waals and Hydrogen Bonds
Matter exists in the forms of elements and compounds, with each of them comprising of molecules and atoms.
There are inter-atomic and intermolecular forces respectively, called as bonds. These bonds determine the behaviour of the molecules, more precisely its stability. Van der Waals forces and hydrogen bonds are two types of these bonds. There are many differences between these bonds, which have been explained in this article.
Hydrogen bonds always include hydrogen which is always connected to a highly electronegative atom in the same molecule, or other. Hydrogen bonding always occurs between two permanent dipoles, and is much stronger that the Van der Waals forces, which on the other hand, are found between the combination of either two permanent dipoles, dipole- induced dipole or two induced dipoles – meaning that they can be present without the presence of a permanent dipole.
Instructions
-
1
Van der Waals forces
For a bond to occur within a molecule there should be opposite charges on the neighbouring atoms. However in some molecules, like Cl2 and H2, both the atoms are same and so are their charges. Thus there is no charge separation.
In these molecules however, the electrons are always moving and when the electron cloud moves towards one end of the molecule, a dipole is created at the other end, which can attract the electrons in the neighbouring molecule, which is undergoing a similar process. The force of attraction between these induced dipoles is called the Van der Waals forces. This is not the only scenario, in which these forces can occur; they can also occur between a permanent dipole and an induced one, or two permanent dipoles.
Image courtesy: ndt-ed.org
-
2
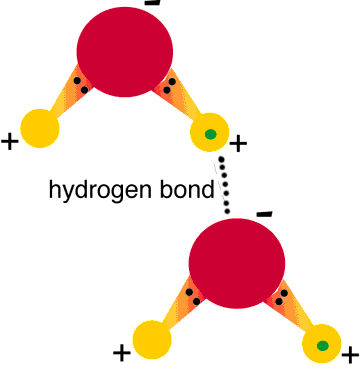
Hydrogen Bonds
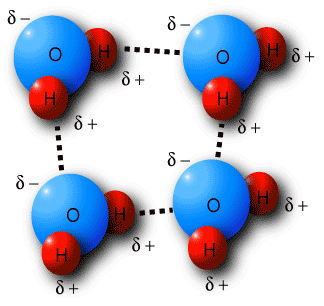
This type of bonding is much stronger than the bonds between other permanent dipoles and is found in molecules where, hydrogen has a bond with highly electronegative atoms like oxygen, hydrogen and nitrogen.
When hydrogen forms a bond with these atoms, their electro-negativity ensures that all the electrons are attracted towards them, leaving a negative charge on them, and a positive one on the hydrogen atom. In case there is a similar molecule in the vicinity, hydrogen of this molecule will be attracted to the electronegative atom of the other, and thus a bond will be formed.
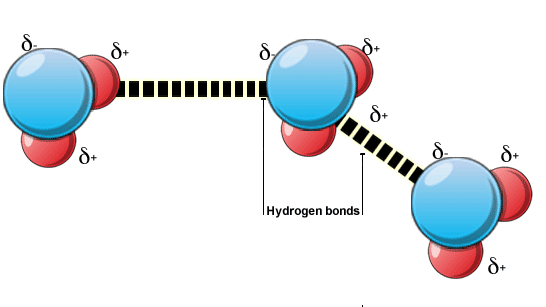
A prominent example of hydrogen bonding is apparent in water. A single water molecule can form up to four hydrogen bonds with other molecule.
Image courtesy: academic.brooklyn.cuny.edu