How to Prepare Sulphuric Acid Industrially
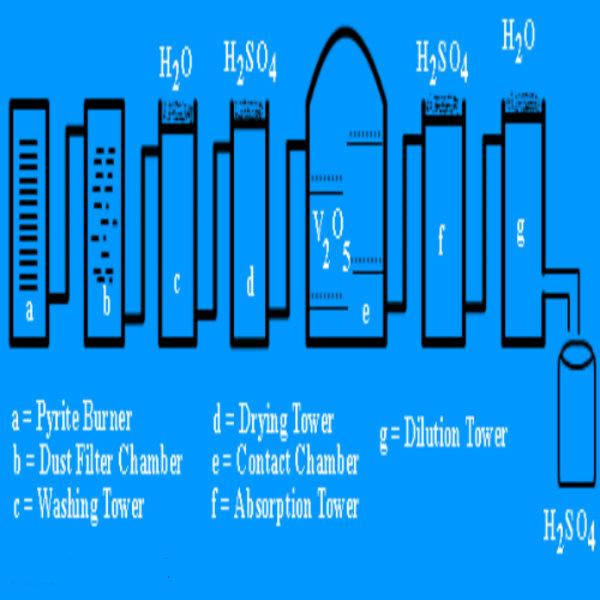
If you are a Chemistry student then you must be familiar with Sulphuric Acid since it is involved in a number of chemical reactions and chemical equations. Sulphuric Acid is also used in the production of various fertilizers such as ammonium sulphate, potassium sulphate and calcium superphosphate. Apart from this, it is also used in the production of soaps, detergents as well as cleaning metals. The other name for Sulphuric Acid is Tetraoxosulphate (VI) acid and it is chemically represented as H2SO4. If you are preparing for your exams and are not able to understand the process of preparing Sulphuric acid, then the following steps will explain how to to do it.
Instructions
-
1
Production of Sulphur Dioxide
The very first step in manufacturing Sulphuric Acid is to produce Sulphur Dioxide gas. It serves as a raw material in production of Sulphuric Acid and is formed by burning Sulphur in presence of oxygen.
Sulphur + Oxygen Sulphur Dioxide
S + O2 ---------------------- SO2 -
2
Purification of Sulphur Dioxide
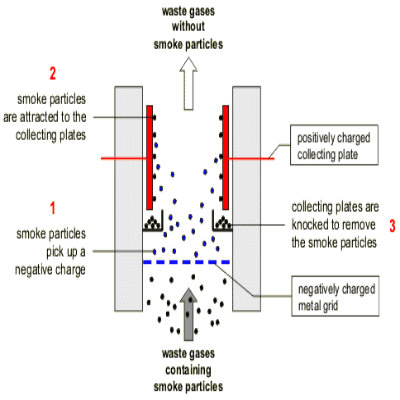
After production, Sulphur Dioxide is purified in order to remove impurities such as arsenic compounds, which may poison the catalyst. This purification is done by passing Sulphur Dioxide through an Electrostatic Dust Precipitator. The dust particles are then charged and separated by getting attracted towards the oppositely charged plates.
-
3
Catalytic conversion of Sulphur Dioxide into Sulphur Trioxide
Sulphur Dioxide and air is washed, dried and passed through the heated catalyst chamber of Vanadium (V) Oxide at a temperature of 450 degrees and pressure of 2 to 3 atmospheres. This chemical reaction is reversible, highly exothermic and yields 98% conversion.
Sulphur + Oxygen ⇌ Sulphur
Dioxide Trioxide
2SO2 + O2 ⇌ 2SO3
-
4
Dissolving Sulphur Trioxide in Concentrated Sulphuric Acid
Sulphur Trioxide gas produced is then dissolved into concentrated Sulphuric Acid to yield Oleum which is also known as fuming Sulphuric Acid.
Sulphur + Conc. Sulphuric Oleum
Trioxide Acid
SO3 + H2SO4 ------------------------ H2S2O7
-
5
Formation of Sulphuric Acid
Finally the Oleum produced is diluted with water to obtain Sulphuric Acid. The chemical reaction is as follows:
Oleum + Water -------------------------- Sulphuric Acid
H2S2O7 + H2O -------------------------- 2H2SO4





