Difference Between Ethanol and Alcohol
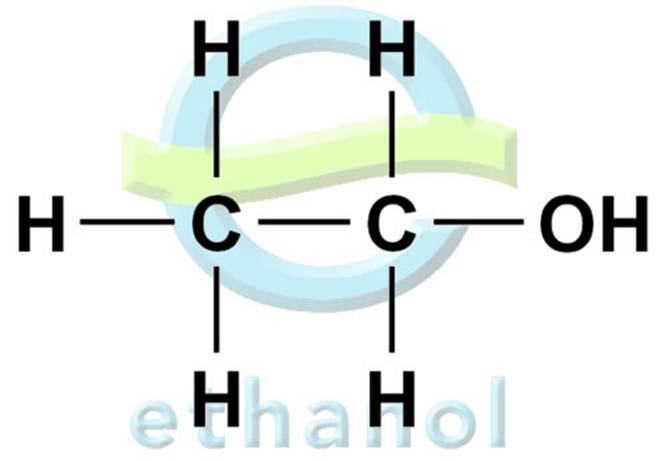
Ethanol and alcohol are both organic compounds with a hydroxyl (OH) ion attached directly to a carbon atom. The only difference between the two is that the former is a specific organic compound while the latter denotes the family of organic compounds with an “OH functional group” – in simple words ethanol is one of the types of alcohol.
Alcohols have been in use since thousands of years now, while ethanol was introduced by Persians in the 19th century. The former have a stinging odour, while the latter has a sweeter, fruit like odour.
Only methanol and propanol are soluble in water, while higher chain alcohols like butanol and pentanol are all insoluble. On the contrary, Ethanol mixes together with water completely. Those alcohols which are insoluble in water however, are found to be miscible with oil.
Both ethanol and other alcohols have high boiling points, due to the presence of hydrogen bonds between their molecules. Ethanol has a boiling point over 78 degrees Celsius.
Furthermore, ethanol is the only consumable alcohol, but it is also mixed with water to decrease its concentration. The ethanol content in alcoholic beverages ranges from 5% to 40%. Pure alcohol on the other hand, contains no water and is not consumable.
Ethanol also has clinical uses and is widely used as an antiseptic to sanitize the skin before injections. No other alcohol is used for medicinal purposes.
Instructions
-
1
Alcohol
Alcohols make a family of organic hydrocarbons, with a hydroxyl functional group directly attached with one carbon of the C-C chain. They have a general formula - CnH2n+1OH, where n stands for number of carbons present in a particular alcohol. Methyl alcohol (CH3OH), Propyl Alcohol (C3H7OH) and Butyl alcohol (C4H9OH) serve as common examples of alcohols.
Alcohols are broadly divided into three types, primary, secondary and tertiary, depending upon the number of carbon atoms linked to the carbon atom directly attached to the hydroxyl group. -
2
Ethanol
Ethanol or Ethyl Alcohol is an example of primary alcohols with a chemical formula CH3CH2OH or C2H5OH. Alcohols are formed by fermentation of glucose, produced from the hydrolysis of starch, using enzymes in the presence of yeast as a catalyst.







