Salt Water Conductivity Experiment
You are in search of ideas for you middle school science project that you have to submit in the next few days. Despite the fact you have tried a number of ideas but you always end up either finding someone else working on the same idea or the expense involving in the project holds you back.
If you’re looking for demonstration of a simple scientific rule or concept for which most of the equipments are probably available at home, Salt Water Conductivity Experiment can be one good option. If you need to buy anything for the demonstration by any chance, that may not be too expensive.
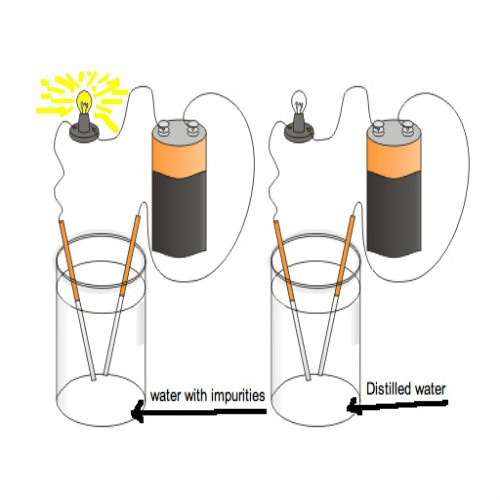
In this experiment you just have to prove that pure water is not a good electricity conductor but addition of salt (Sodium Chloride) makes it ionized and hence conduct electric current.
Things Required:
– Drinking Water Glass
– Battery 4 to 5 Volts
– Voltmeter
– 3 Wires with Crocodiles
– 2 Copper Electrodes
– Distilled Water
– Table Salt (Sodium Chloride)
– Teaspoon
Instructions
-
1
Making Connection
With the help of a crocodile wire, connect the red terminal of the voltmeter with one of the two copper electrodes. In the same way, connect the black terminal (COM) of the voltmeter to the negative terminal of the battery with the second crocodile ended wire.

-
2
Completing Connection
Now using the third wire with crocodile extremity, connect the positive terminal of the battery with the other copper plate. This completes the wire connection for the experiment. -
3
Inserting Copper Electrodes
After that, fill the glass with distilled water and then immerse the two copper electrodes in it without letting them come in contact with each other. It will be observed that there will be negligible deflection shown in the voltmeter. -
4
Removing Copper Electrodes
Remove the copper electrodes from the glass and add some salt in to the water. Stir thoroughly with the help of tea spoon to let the salt dissolved properly. -
5
Placing Copper Electrodes in Salted Water
Now again immerse the copper electrodes in the salt solution. It will be noticed that some deflection is shown in the voltmeter showing that addition of salt ionizes pure water and allows conduction of electric current.