Difference Between Valency and Oxidation State
There are 118 known elements, from Hydrogen (1) to ununoctium (118), and of these only 98 elements occur naturally, while the rest have been synthesised or produced artificially in the laboratory. Only the group VIII A members have a complete outermost shell (i.e., they possess the required number of electrons in their outermost shell), while the rest of the elements lose or gain electrons to complete their outermost shell.
This tendency of an element to gain or lose electrons in order to complete its outermost shell is known as its valency. Meanwhile, the oxidation state is the apparent charge on an atom, or the degree of oxidation of an atom in a chemical compound, and also involves the gain or loss of electrons.
While both valency and oxidation state involve the addition and removal of electrons, they are two distinct terms. The former is the maximum number of electrons which an atom can gain or lose, while the latter is the actual number of electrons which the element gains or loses in a chemical compound.
Furthermore, valency is the property exhibited by an isolated atom, while oxidation state is the property exhibited by a bonded atom. While both valency and oxidation state involve the gain or loss of electrons from an atom of the same element, both of them may or may not be equal.
The valency of an element remains the same, while the oxidation state of an element is different for different molecules. Moreover, the oxidation of an atom can be fractional, but the valency can never be fractional, and always has a value in whole numbers.
Instructions
-
1
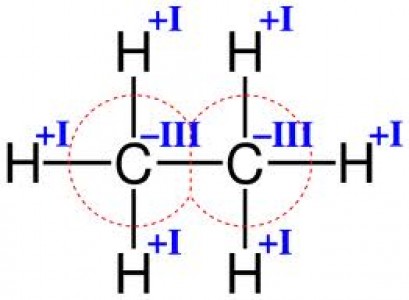
Valency
According to the definition given by the International Union of Pure and Applied Chemistry (IUPAC), valency is the “maximum number of univalent atoms that may combine with the atom”. In simple words, the valency of an atom is represented by the number of bonds it can form. The valency of an element is determined by the number of electrons present in its valence shell. During the chemical bond formation, the atom either loses these valence electrons or adds more electrons to the valence shell to stabilize it by completing the outermost shell. Valency is a whole number value that can be positive (when losing electrons) or negative (when gaining electrons).
Image courtesy: chelationtherapyonline.com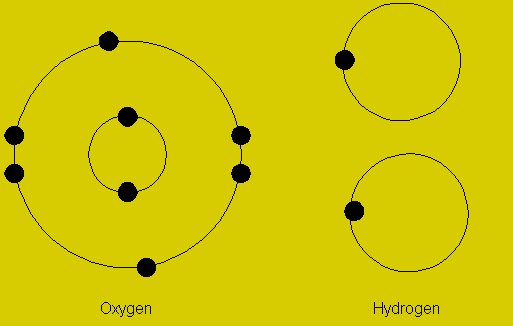
-
2
Oxidation State
According to the IUPAC definition, oxidation state is “a measure of the degree of oxidation of an atom in a substance. It is defined as the charge an atom might be imagined to have”. The oxidation state of an atom is subject to the chemical reaction it is undergoing. If there is an increase in the oxidation state of an atom it is said to be oxidized, and in the opposite case, the atom is said to be reduced.
Image courtesy: intechopen.com